Atoms
-
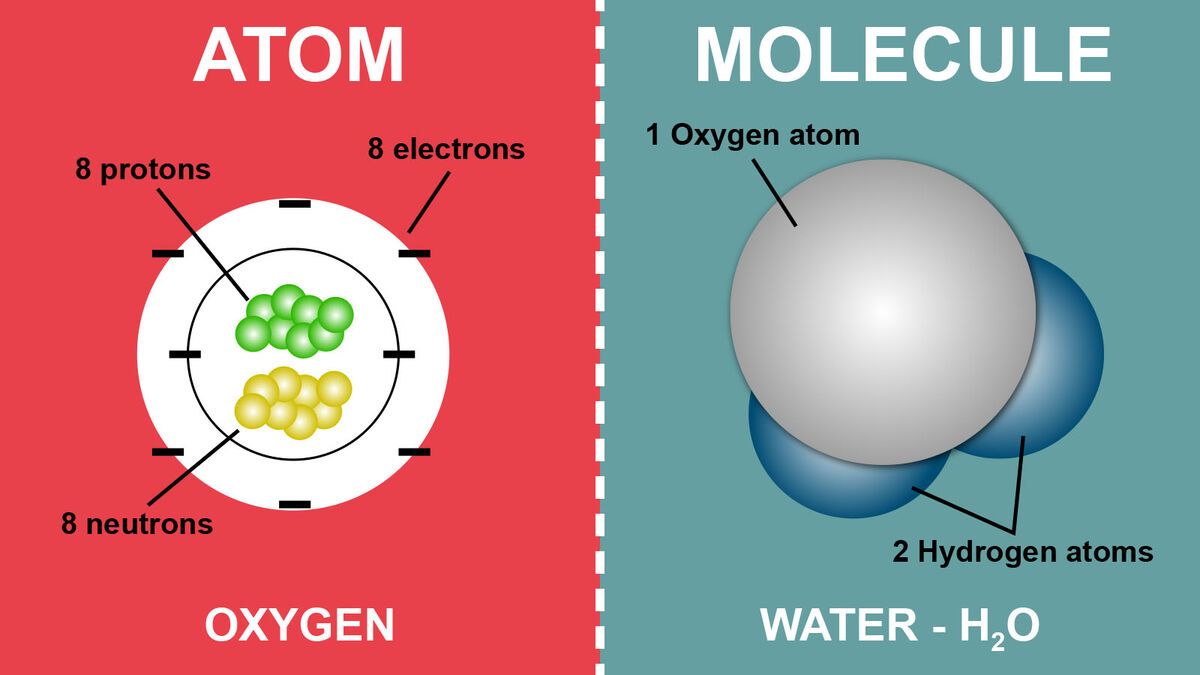
The Fundamental Building Blocks: Atoms are the smallest units of matter that retain the properties of an element. They are incredibly small, with diameters measured in the range of a tenth of a nanometer.
-
Structure of an Atom:
- Nucleus: The central, dense core containing:
- Protons: Positively charged particles. The number of protons determines the element.
- Neutrons: Neutral particles contributing to an atom's mass.
- Electron Cloud: A region around the nucleus where negatively charged electrons rapidly orbit.
-
Elements: Pure substances consisting of only one type of atom. Examples: carbon, oxygen, gold, helium.
-
Atomic Number: The number of protons in an atom's nucleus. Defines the element.
-
Atomic Mass: The total mass of protons, neutrons, and electrons in an atom (electrons contribute a negligible amount).
Molecules
- Atoms Bonded Together: Molecules form when two or more atoms join together through chemical bonds. They can be composed of atoms of the same element (like oxygen gas - O2) or different elements (like water - H2O).
- Chemical Bonds: The forces that hold atoms together in molecules. The main types are:
- Covalent Bonds: Covalent Bonds: Sharing is Caring
-
Formation: Covalent bonds form when atoms share pairs of electrons. This sharing occurs between atoms with similar electronegativities, commonly between nonmetal atoms.
-
The Goal: Atoms share electrons to achieve a stable electron configuration, often similar to that of noble gases (with full outer electron shells). This stability makes covalent bonds very strong.
-
Types of Covalent Bonds:
- Single Bond: One pair of electrons is shared (Example: H-H in a hydrogen molecule)
- Double Bond: Two pairs of electrons are shared (Example: O=O in an oxygen molecule)
- Triple Bond: Three pairs of electrons are shared (Example: N≡N in a nitrogen molecule)
-
Molecules: Covalent bonds result in the formation of discrete molecules, with a defined number of atoms held together.
Example: Water (H2O)
- An oxygen atom shares one electron with each of two hydrogen atoms.
- Each hydrogen shares its single electron with oxygen.
- This results in a stable molecule with oxygen achieving a full outer shell of 8 electrons, and each hydrogen achieving a full shell of 2 electrons.
Properties of Covalent Compounds
- State of matter: Covalent compounds can exist as solids, liquids, or gases at room temperature.
- Low melting and boiling points: The forces between individual covalent molecules are weaker than in ionic compounds, leading to lower melting and boiling points.
- Poor electrical conductivity: Covalently bonded molecules do not contain free-moving charged particles, making them poor conductors.
- Varied Properties: Covalent compounds can exhibit a diverse range of properties based on their molecular structures.
Importance of Covalent Bonds
-
Foundation of Life: Covalent bonds form the backbone of the essential biomolecules:
- Carbohydrates
- Lipids
- Proteins
- Nucleic Acids (DNA and RNA)
-
Vast Number of Compounds: The ability of atoms like carbon to form multiple covalent bonds leads to a vast array of organic compounds, forming the basis for a huge portion of the materials we interact with.
Let me know if you'd like:
- Visual diagrams of covalent bond formations
- A comparison table between covalent and ionic bonds
- Examples of important covalent compounds
-
- Ionic Bonds:
Formation: Ionic bonds form between atoms with significantly different electronegativities (attraction for electrons).
- Metals tend to have low electronegativity and readily lose electrons, becoming positively charged ions (cations).
- Non-metals have high electronegativity and readily gain electrons, becoming negatively charged ions (anions).
-
The Bond: Ionic bonds result from the electrostatic attraction between these oppositely charged ions. Imagine it like magnets with opposite poles sticking together.
-
Crystal Lattices: Ionic compounds don't usually form individual molecules. Rather, the attraction between the positive and negative ions leads to the formation of large, organized 3D structures called crystal lattices.
- Sodium (Na) loses one electron to become a positively charged sodium ion (Na+).
- Chlorine (Cl) gains one electron to become a negatively charged chloride ion (Cl-).
- The electrostatic attraction between Na+ and Cl- pulls them together, forming an ionic bond and creating the compound sodium chloride (table salt).
- Solid at room temperature: The strong electrostatic forces in ionic bonds make ionic compounds solid at room temperature.
- High melting and boiling points: It takes a significant amount of energy to break the strong bonds in an ionic crystal lattice.
- Conductive when molten or dissolved: When melted or dissolved, the ions in an ionic compound are free to move, allowing them to carry an electrical current.
- Often brittle: While the bonds within the lattice are strong, shifting the alignment of the ions can cause repulsion, making them shatter.
- Biological Roles: Ions formed through ionic bonding play crucial roles in biological processes. For example:
- Sodium (Na+) and potassium (K+) ions are essential for nerve and muscle function.
- Calcium ions (Ca2+) are vital for bones and teeth.
- Common Compounds: Many familiar substances are formed with ionic bonds:
- Table salt (NaCl)
- Baking soda (Sodium bicarbonate - NaHCO3)
- Calcium carbonate (CaCO3) – found in chalk and limestone
- Molecular Formula: Represents the elements and their quantity within a molecule (e.g., H2O, CO2).
Example: Sodium Chloride (NaCl)
Properties of Ionic Compounds
Importance of Ionic Bonds
Key Differences Between Atoms and Molecules
- Size: Molecules are generally larger than atoms as they consist of multiple atoms bonded together.
- Stability: Atoms of certain elements can exist independently, while molecules are the smallest independent unit for many substances.
- Composition: Atoms are made of protons, neutrons, and electrons. Molecules are made of atoms held together by chemical bonds.
Importance of Atoms and Molecules
Atoms
- Building Blocks of Matter: Atoms are the fundamental units from which everything around us is constructed. Understanding atoms is essential to understanding the composition and behavior of all matter.
- Elements and the Periodic Table: The diversity of elements, each composed of a unique type of atom, gives rise to the rich variety of materials we find in the universe. The Periodic Table organizes these elements based on their atomic structure, providing a powerful framework for understanding chemical properties and reactivity.
- Chemical Reactions: Chemical reactions involve the rearrangement, breaking, and forming of bonds between atoms. These processes are the basis for everything from the metabolism within our bodies to the creation of complex industrial materials.
Molecules
- Foundation of Life: The complex molecules that make up living organisms (carbohydrates, lipids, proteins, and nucleic acids) are all built upon arrangements of atoms. Understanding molecular structure is critical to comprehending cellular processes, genetic inheritance, and biological functions.
- Diversity and Properties: The types of atoms and how they bond within molecules dictate the properties of substances. This leads to a vast range of materials, from water essential for life to the plastics with unique properties we use every day.
- Medicine and Technology: Understanding molecular structure and bonding allows scientists to design new drugs, develop advanced materials, and create innovative technologies that improve our everyday lives.
Overall Importance
Atoms and molecules lie at the heart of chemistry and biology. From the simplest substances to the most complex living things, their structure and interaction form the basis of our understanding of the natural world. Key areas where this knowledge is applied include:
- Medicine: The design of drugs and treatments relies on how molecules interact within the body.
- Materials Science: Creating materials with specific properties depends on our understanding of atomic and molecular structure.
- Environmental Science: Analyzing pollutants, understanding climate change, and developing sustainable technologies hinges on a molecular-level view.
Visuals
Images and diagrams of atoms and various molecules (like water, methane, carbon dioxide) would greatly enhance the understanding of these concepts.